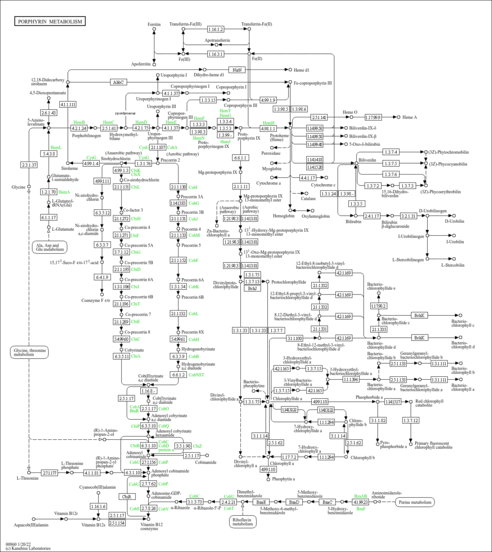
Showing metabocard for Heme (HMDB0003178)
Only showing the first 10 proteins. There are 170 proteins in total.
Enzymes
- General function:
- Involved in 5-aminolevulinate synthase activity
- Specific function:
- Not Available
- Gene Name:
- ALAS2
- Uniprot ID:
- P22557
- Molecular weight:
- 64632.86
- General function:
- Involved in 5-aminolevulinate synthase activity
- Specific function:
- Not Available
- Gene Name:
- ALAS1
- Uniprot ID:
- P13196
- Molecular weight:
- 70580.325
- General function:
- Involved in succinate dehydrogenase activity
- Specific function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHC
- Uniprot ID:
- Q99643
- Molecular weight:
- 16650.185
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Not Available
- Gene Name:
- AOX1
- Uniprot ID:
- Q06278
- Molecular weight:
- 147916.735
- General function:
- Involved in heme oxygenase (decyclizing) activity
- Specific function:
- Heme oxygenase cleaves the heme ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of heme oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed. Heme oxygenase 2 could be implicated in the production of carbon monoxide in brain where it could act as a neurotransmitter.
- Gene Name:
- HMOX2
- Uniprot ID:
- P30519
- Molecular weight:
- 36032.615
Reactions
| Heme + AH(2) + Oxygen → Biliverdin + Fe2+ + CO + A + Water | details |
- General function:
- Involved in iron ion binding
- Specific function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q) (By similarity).
- Gene Name:
- SDHD
- Uniprot ID:
- O14521
- Molecular weight:
- 17042.82
- General function:
- Involved in oxidoreductase activity
- Specific function:
- This enzyme is required for electron transfer from NADP to cytochrome P450 in microsomes. It can also provide electron transfer to heme oxygenase and cytochrome B5.
- Gene Name:
- POR
- Uniprot ID:
- P16435
- Molecular weight:
- 77047.575
- General function:
- Involved in heme oxygenase (decyclizing) activity
- Specific function:
- Heme oxygenase cleaves the heme ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of heme oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed.
- Gene Name:
- HMOX1
- Uniprot ID:
- P09601
- Molecular weight:
- 32818.345
Reactions
| Heme + AH(2) + Oxygen → Biliverdin + Fe2+ + CO + A + Water | details |
- General function:
- Involved in uroporphyrinogen decarboxylase activity
- Specific function:
- Catalyzes the decarboxylation of four acetate groups of uroporphyrinogen-III to yield coproporphyrinogen-III.
- Gene Name:
- UROD
- Uniprot ID:
- P06132
- Molecular weight:
- 40786.58
- General function:
- Involved in coproporphyrinogen oxidase activity
- Specific function:
- Key enzyme in heme biosynthesis. Catalyzes the oxidative decarboxylation of propionic acid side chains of rings A and B of coproporphyrinogen III.
- Gene Name:
- CPOX
- Uniprot ID:
- P36551
- Molecular weight:
- 50151.605
Transporters
- General function:
- Involved in transmembrane transport
- Specific function:
- Has been shown to act both as an intestinal proton- coupled high-affinity folate transporter and as an intestinal heme transporter which mediates heme uptake from the gut lumen into duodenal epithelial cells. The iron is then released from heme and may be transported into the bloodstream. Dietary heme iron is an important nutritional source of iron. Shows a higher affinity for folate than heme
- Gene Name:
- SLC46A1
- Uniprot ID:
- Q96NT5
- Molecular weight:
- 49770.0
Only showing the first 10 proteins. There are 170 proteins in total.