| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:08 UTC |
|---|
| HMDB ID | HMDB0001231 |
|---|
| Secondary Accession Numbers | - HMDB0006264
- HMDB01231
- HMDB06264
|
|---|
| Metabolite Identification |
|---|
| Common Name | 27-Deoxy-5b-cyprinol |
|---|
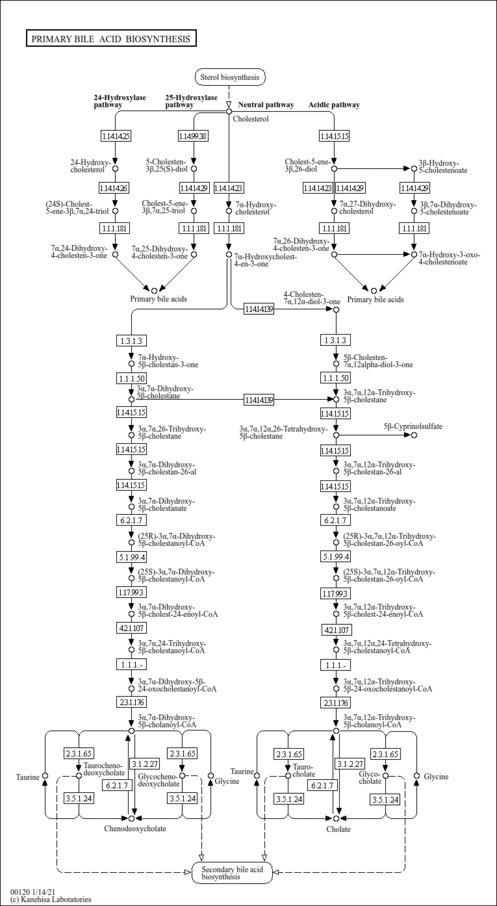
| Description | 27-Deoxy-5b-cyprinol is an intermediate in Bile acid synthesis pathway, in a sequence of reactions catalyzed by sterol 27-hydroxylase (CYP27) in the oxidation of 5 beta-cholestane-3 alpha,7 alpha,12 alpha,27-tetrol into 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid (PMID: 8496170 ). 5 beta-cholestane-3 alpha,7 alpha,12 alpha,25-tetrol 3-glucuronide, a metabolite of 27-Deoxy-5b-cyprinol, is the major bile alcohol component in serum from cerebrotendinous xanthomatosis patients (PMID: 7920441 ). |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCCC(C)CO InChI=1S/C27H48O4/c1-16(15-28)6-5-7-17(2)20-8-9-21-25-22(14-24(31)27(20,21)4)26(3)11-10-19(29)12-18(26)13-23(25)30/h16-25,28-31H,5-15H2,1-4H3/t16?,17-,18+,19-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha,5beta,7alpha,12alpha)-Cholestane-3,7,12,26-tetrol | ChEBI | | 3alpha,7alpha,12alpha,26-Tetrahydroxy-5beta-cholestane | ChEBI | | 5beta-Cholestane 3alpha,7alpha,12alpha,27-tetrol | ChEBI | | 5beta-Cholestane-3alpha,7alpha,12alpha,26-tetraol | ChEBI | | Cholestane-3,7,12,26(27)-tetrol | ChEBI | | Cholestane-3,7,12,26-tetrol | ChEBI | | Cholestane-3,7,12,27-tetrol | ChEBI | | 5beta-Cholestane-3alpha,7alpha,12alpha,26-tetrol | Kegg | | (3a,5b,7a,12a)-Cholestane-3,7,12,26-tetrol | Generator | | (3Α,5β,7α,12α)-cholestane-3,7,12,26-tetrol | Generator | | 3a,7a,12a,26-Tetrahydroxy-5b-cholestane | Generator | | 3Α,7α,12α,26-tetrahydroxy-5β-cholestane | Generator | | 5b-Cholestane 3a,7a,12a,27-tetrol | Generator | | 5Β-cholestane 3α,7α,12α,27-tetrol | Generator | | 5b-Cholestane-3a,7a,12a,26-tetraol | Generator | | 5Β-cholestane-3α,7α,12α,26-tetraol | Generator | | 5b-Cholestane-3a,7a,12a,26-tetrol | Generator | | 5Β-cholestane-3α,7α,12α,26-tetrol | Generator | | (25R)-5-beta-Cholestane-3-alpha,7-alpha,12-alpha,26-tetraol | HMDB | | 5-b-Cholestane-3a-7-tetraol | HMDB | | 5-beta-Cholestane-3-alpha-7-alpha-12-alpha-26-tetraol | HMDB | | 5-beta-Cholestane-3a-7-tetraol | HMDB | | 5beta-Cholestane-3alpha,7alpha,12alpha,27-tetraol | HMDB | | 5beta-Cholestane-3alpha,7alpha,12alpha,27-tetrol | HMDB | | 5beta-Cholestane-3alpha,7alpha,12alpha,27alpha-tetrol | HMDB | | 5 beta-Cholestane-3alpha,7alpha,12alpha,26-tetrol | HMDB | | 5-CTTL | HMDB | | Cholestane-3,7,12,26-tetrol, (3alpha,5alpha,7alpha,12alpha)-isomer | HMDB |
| Show more...
|---|
| Chemical Formula | C27H48O4 |
|---|
| Average Molecular Weight | 436.6676 |
|---|
| Monoisotopic Molecular Weight | 436.355260024 |
|---|
| IUPAC Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-14-[(2R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-5,9,16-triol |
|---|
| Traditional Name | 5-cttl |
|---|
| CAS Registry Number | 862-53-3 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCCC(C)CO |
|---|
| InChI Identifier | InChI=1S/C27H48O4/c1-16(15-28)6-5-7-17(2)20-8-9-21-25-22(14-24(31)27(20,21)4)26(3)11-10-19(29)12-18(26)13-23(25)30/h16-25,28-31H,5-15H2,1-4H3/t16?,17-,18+,19-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | XJZGNVBLVFOSKJ-XZULNKEGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as tetrahydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears four hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Tetrahydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 26-hydroxysteroid
- Tetrahydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 12-hydroxysteroid
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- Fatty alcohol
- Fatty acyl
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 27-Deoxy-5b-cyprinol,1TMS,isomer #1 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 3494.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TMS,isomer #2 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 3549.0 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 3542.2 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O)CO[Si](C)(C)C | 3545.6 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3441.2 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #2 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 3466.8 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 3444.4 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O)CO[Si](C)(C)C | 3504.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #5 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 3478.4 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TMS,isomer #6 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O)CO[Si](C)(C)C | 3500.5 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3445.8 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TMS,isomer #2 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3430.8 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 3450.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O)CO[Si](C)(C)C | 3432.2 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,4TMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C)CO[Si](C)(C)C | 3445.7 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TBDMS,isomer #1 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3707.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TBDMS,isomer #2 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 3759.4 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TBDMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 3765.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,1TBDMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 3777.5 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 3874.7 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #2 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3908.9 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3874.5 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 3979.4 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #5 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 3913.7 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,2TBDMS,isomer #6 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 3946.1 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TBDMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4104.0 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TBDMS,isomer #2 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4077.9 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TBDMS,isomer #3 | CC(CO)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4094.2 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,3TBDMS,isomer #4 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O)CO[Si](C)(C)C(C)(C)C | 4125.4 | Semi standard non polar | 33892256 | | 27-Deoxy-5b-cyprinol,4TBDMS,isomer #1 | CC(CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C)CO[Si](C)(C)C(C)(C)C | 4315.4 | Semi standard non polar | 33892256 |
| Show more...
|---|