| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-05-30 20:56:00 UTC |
|---|
| HMDB ID | HMDB0000715 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Kynurenic acid |
|---|
| Description | Kynurenic acid (KYNA) is a well-known endogenous antagonist of the glutamate ionotropic excitatory amino acid receptors N-methyl-D-aspartate (NMDA), alphaamino-3-hydroxy-5-methylisoxazole-4-propionic acid and kainate receptors and of the nicotine cholinergic subtype alpha 7 receptors. KYNA neuroprotective and anticonvulsive activities have been demonstrated in animal models of neurodegenerative diseases. Because of KYNA's neuromodulatory character, its involvement has been speculatively linked to the pathogenesis of a number of neurological conditions including those in the ageing process. Different patterns of abnormalities in various stages of KYNA metabolism in the CNS have been reported in Alzheimer's disease, Parkinson's disease and Huntington's disease. In HIV-1-infected patients and in patients with Lyme neuroborreliosis a marked rise of KYNA metabolism was seen. In the ageing process KYNA metabolism in the CNS of rats shows a characteristic pattern of changes throughout the life span. A marked increase of the KYNA content in the CNS occurs before the birth, followed by a dramatic decline on the day of birth. A low activity was seen during ontogenesis, and a slow and progressive enhancement occurs during maturation and ageing. This remarkable profile of KYNA metabolism alterations in the mammalian brain has been suggested to result from the development of the organisation of neuronal connections and synaptic plasticity, development of receptor recognition sites, maturation and ageing. There is significant evidence that KYNA can improve cognition and memory, but it has also been demonstrated that it interferes with working memory. Impairment of cognitive function in various neurodegenerative disorders is accompanied by profound reduction and/or elevation of KYNA metabolism. The view that enhancement of CNS KYNA levels could underlie cognitive decline is supported by the increased KYNA metabolism in Alzheimer's disease, by the increased KYNA metabolism in down's syndrome and the enhancement of KYNA function during the early stage of Huntington's disease. Kynurenic acid is the only endogenous N-methyl-D-aspartate (NMDA) receptor antagonist identified up to now, that mediates glutamatergic hypofunction. Schizophrenia is a disorder of dopaminergic neurotransmission, but modulation of the dopaminergic system by glutamatergic neurotransmission seems to play a key role. Despite the NMDA receptor antagonism, kynurenic acid also blocks, in lower doses, the nicotinergic acetycholine receptor, i.e., increased kynurenic acid levels can explain psychotic symptoms and cognitive deterioration. Kynurenic acid levels are described to be higher in the cerebrospinal fluid (CSF) and in critical central nervous system (CNS) regions of schizophrenics as compared to controls. (PMID: 17062375 , 16088227 ). KYNA has also been identified as a uremic toxin according to the European Uremic Toxin Working Group (PMID: 22626821 ). | Read more...
|---|
| Structure | OC(=O)C1=CC(=O)C2=CC=CC=C2N1 InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14) |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-2-chinolincarbonsaeure | ChEBI | | 4-Hydroxy-2-quinolinecarboxylic acid | ChEBI | | 4-Hydroxyquinaldic acid | ChEBI | | 4-Hydroxyquinaldinic acid | ChEBI | | Kynurenate | ChEBI | | Kynurensaeure | ChEBI | | 4-Hydroxy-2-quinolinecarboxylate | Generator | | 4-Hydroxyquinaldate | Generator | | 4-Hydroxyquinaldinate | Generator | | 2-Carboxy-4-hydroxyquinoline | HMDB | | 4-Hydroxy-quinaldate | HMDB | | 4-Hydroxy-quinaldic acid | HMDB | | 4-Hydroxyquinoline-2-carboxylate | HMDB | | 4-Hydroxyquinoline-2-carboxylic acid | HMDB | | Quinurenic acid | HMDB | | Acid, kynurenic | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H7NO3 |
|---|
| Average Molecular Weight | 189.1675 |
|---|
| Monoisotopic Molecular Weight | 189.042593095 |
|---|
| IUPAC Name | 4-oxo-1,4-dihydroquinoline-2-carboxylic acid |
|---|
| Traditional Name | acid, kynurenic |
|---|
| CAS Registry Number | 492-27-3 |
|---|
| SMILES | OC(=O)C1=CC(=O)C2=CC=CC=C2N1 |
|---|
| InChI Identifier | InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14) |
|---|
| InChI Key | HCZHHEIFKROPDY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as quinoline carboxylic acids. These are quinolines in which the quinoline ring system is substituted by a carboxyl group at one or more positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinoline carboxylic acids |
|---|
| Direct Parent | Quinoline carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinoline-2-carboxylic acid
- Dihydroquinolone
- Dihydroquinoline
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Azacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 280 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Kynurenic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2[NH]1 | 2044.7 | Semi standard non polar | 33892256 | | Kynurenic acid,1TMS,isomer #2 | C[Si](C)(C)N1C(C(=O)O)=CC(=O)C2=CC=CC=C21 | 2103.8 | Semi standard non polar | 33892256 | | Kynurenic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C | 2150.9 | Semi standard non polar | 33892256 | | Kynurenic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C | 2135.8 | Standard non polar | 33892256 | | Kynurenic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C | 2267.1 | Standard polar | 33892256 | | Kynurenic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2[NH]1 | 2290.7 | Semi standard non polar | 33892256 | | Kynurenic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N1C(C(=O)O)=CC(=O)C2=CC=CC=C21 | 2319.0 | Semi standard non polar | 33892256 | | Kynurenic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C(C)(C)C | 2554.6 | Semi standard non polar | 33892256 | | Kynurenic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C(C)(C)C | 2530.4 | Standard non polar | 33892256 | | Kynurenic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1=CC(=O)C2=CC=CC=C2N1[Si](C)(C)C(C)(C)C | 2516.2 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Kynurenic acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0159-1985000000-f1c74ffc481c3d0faaa2 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Kynurenic acid GC-MS (2 TMS) | splash10-0159-3895000000-6ba2750c5a9d9754abcc | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Kynurenic acid EI-B (Non-derivatized) | splash10-0159-0498000000-3db9df2c3d8456d7ba63 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Kynurenic acid GC-EI-TOF (Non-derivatized) | splash10-0159-1985000000-f1c74ffc481c3d0faaa2 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Kynurenic acid GC-MS (Non-derivatized) | splash10-0159-3895000000-6ba2750c5a9d9754abcc | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Kynurenic acid GC-EI-TOF (Non-derivatized) | splash10-0159-1895000000-e25b66b0b42d1928cc67 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-02ga-1900000000-8c67ab2acdc02b88af2b | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9740000000-a7c5c056d48055e0d44f | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Kynurenic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-000i-0900000000-7a5c54ced5b83f291e67 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0006-0900000000-e6b451ee354ed2717160 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-000i-9500000000-887e5157431b2908f31e | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-0006-1900000000-06f7059894cc245cb344 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-0006-0900000000-89a88f44f08b8b68d5c1 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-qTof , Positive-QTOF | splash10-014l-0900000000-3a256522dd8694fe04c0 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-000f-0900000000-a4b194b85fef656b9b65 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-0006-0900000000-7862c532805b6036dc67 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-0006-0900000000-5fe3171fdaece30b78c8 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-0006-0900000000-df3b5b36289798800ad8 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-0006-0900000000-d4c11672b9479b2cd4b6 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , negative-QTOF | splash10-0006-0900000000-89a88f44f08b8b68d5c1 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid Linear Ion Trap , negative-QTOF | splash10-0006-0900000000-9c26ffa9c04aa03d60f5 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid Linear Ion Trap , negative-QTOF | splash10-0006-0900000000-6a8662ef19d900499a55 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid , negative-QTOF | splash10-0006-0900000000-d69a430b0eb34f38ad54 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , positive-QTOF | splash10-0006-0900000000-d0ef7c769d4985e85667 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , positive-QTOF | splash10-01ox-0900000000-2a593c8017254be4a387 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , positive-QTOF | splash10-01ox-0900000000-59f3559c0b95c9411d2c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Kynurenic acid LC-ESI-QTOF , positive-QTOF | splash10-02tc-0900000000-57dbbfb8e12c60951d77 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 10V, Positive-QTOF | splash10-0006-0900000000-878dcda0ee5ec971fa72 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 20V, Positive-QTOF | splash10-0006-0900000000-92fe5294ec4a771f14e0 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 40V, Positive-QTOF | splash10-0006-1900000000-fa8b36fb6db8e403b711 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 10V, Negative-QTOF | splash10-000i-0900000000-9cede81ef6dfc45f5f7a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 20V, Negative-QTOF | splash10-000f-0900000000-0d5cf046565b518442d5 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Kynurenic acid 40V, Negative-QTOF | splash10-0006-2900000000-2c420ff1e59666527005 | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI+, Adduct: [M+Na]+) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI-, Adduct: [M-H]-) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI+, Adduct: [M+H]+) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Urine
|
|---|
| Tissue Locations | - Brain
- Epidermis
- Fibroblasts
- Placenta
- Prostate
|
|---|
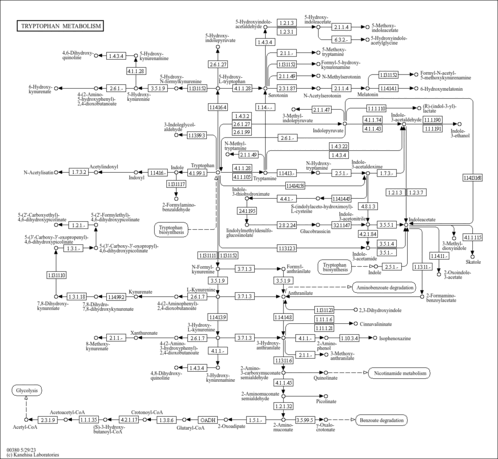
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.023 +/- 0.010 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.03 +/- 0.007 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | <5.291 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.00106 +/- 0.00007 uM | Adult (>18 years old) | Male | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0040 +/- 0.00014 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.07 (0.06-0.08) uM | Adult (>18 years old) | Not Specified | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.0019 (0.0017-0.0021) uM | Adult (>18 years old) | Female | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.001 (0.0009-0.0011) uM | Adult (>18 years old) | Male | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 5.232 +/- 0.546 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 1.34 +/- 0.30 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | - Geigy Scientific ...
- West Cadwell, N.J...
- Basel, Switzerlan...
| details | | Urine | Detected and Quantified | 16.508 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 0.73-1.1 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 1.53 (0.66-4.13) umol/mmol creatinine | Adult (>18 years old) | Not Specified | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 1.6 (0.8-4.2) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.62-0.86 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 4.94 (3.81-7.06) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 2.6(0.9-4.1) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.80 +/- 0.40 uM | Adult (>18 years old) | Both | uremia | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.00133 +/- 0.00009 uM | Adult (>18 years old) | Male | Schizophrenia | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.06 (0.06-0.07) uM | Adult (>18 years old) | Not Specified | Malaria | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 1.23 (0.18-8.40) uM | Not Specified | Not Specified | Anemia | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.21 (0.2-0.3) uM | Not Specified | Not Specified | Malaria | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.07 (0.0-34.1) uM | Not Specified | Not Specified | Tuberculous meningitis | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.05 (0.02-0.10) uM | Not Specified | Not Specified | Convulsions | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.05 (0.01-0.25) uM | Not Specified | Not Specified | Malaria | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Both | Crohns disease | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Both | Ulcerative colitis | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Crohns disease | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Ulcerative colitis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Children (1-13 years old) | Both | Enthesitis-related arthritis | | details | | Urine | Detected and Quantified | 1.881 +/- 0.513 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected and Quantified | 20.976 +/- 3.813 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected and Quantified | 117.043 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Gastroesophageal reflux disease | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Uremia |
|---|
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012 Jul;23(7):1258-70. doi: 10.1681/ASN.2011121175. Epub 2012 May 24. [PubMed:22626821 ]
| | Malaria |
|---|
- Medana IM, Hien TT, Day NP, Phu NH, Mai NT, Chu'ong LV, Chau TT, Taylor A, Salahifar H, Stocker R, Smythe G, Turner GD, Farrar J, White NJ, Hunt NH: The clinical significance of cerebrospinal fluid levels of kynurenine pathway metabolites and lactate in severe malaria. J Infect Dis. 2002 Mar 1;185(5):650-6. Epub 2002 Feb 14. [PubMed:11865422 ]
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [PubMed:12964115 ]
| | Anemia |
|---|
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [PubMed:12964115 ]
| | Tuberculous meningitis |
|---|
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [PubMed:12964115 ]
| | Convulsion |
|---|
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [PubMed:12964115 ]
| | Schizophrenia |
|---|
- Nilsson-Todd LK, Nordin C, Jonsson EG, Skogh E, Erhardt S: Cerebrospinal fluid kynurenic acid in male patients with schizophrenia - correlation with monoamine metabolites. Acta Neuropsychiatr. 2007 Feb;19(1):45-52. doi: 10.1111/j.1601-5215.2006.00170.x. [PubMed:26952797 ]
| | Crohn's disease |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Ulcerative colitis |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
| | Colorectal cancer |
|---|
- Cheng Y, Xie G, Chen T, Qiu Y, Zou X, Zheng M, Tan B, Feng B, Dong T, He P, Zhao L, Zhao A, Xu LX, Zhang Y, Jia W: Distinct urinary metabolic profile of human colorectal cancer. J Proteome Res. 2012 Feb 3;11(2):1354-63. doi: 10.1021/pr201001a. Epub 2011 Dec 28. [PubMed:22148915 ]
- Ni Y, Xie G, Jia W: Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J Proteome Res. 2014 Sep 5;13(9):3857-70. doi: 10.1021/pr500443c. Epub 2014 Aug 14. [PubMed:25105552 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | DB11937 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022200 |
|---|
| KNApSAcK ID | C00026453 |
|---|
| Chemspider ID | 3712 |
|---|
| KEGG Compound ID | C01717 |
|---|
| BioCyc ID | KYNURENATE |
|---|
| BiGG ID | 38234 |
|---|
| Wikipedia Link | Kynurenic_acid |
|---|
| METLIN ID | 5683 |
|---|
| PubChem Compound | 3845 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 18344 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | KYNATE |
|---|
| MarkerDB ID | MDB00000232 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Stone, T. W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends in Pharmacological Sciences (2000), 21(4), 149-154. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Medana IM, Hien TT, Day NP, Phu NH, Mai NT, Chu'ong LV, Chau TT, Taylor A, Salahifar H, Stocker R, Smythe G, Turner GD, Farrar J, White NJ, Hunt NH: The clinical significance of cerebrospinal fluid levels of kynurenine pathway metabolites and lactate in severe malaria. J Infect Dis. 2002 Mar 1;185(5):650-6. Epub 2002 Feb 14. [PubMed:11865422 ]
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [PubMed:12964115 ]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al.: Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115 ( Pt 5):1249-73. [PubMed:1422788 ]
- Parada-Turska J, Rzeski W, Zgrajka W, Majdan M, Kandefer-Szerszen M, Turski W: Kynurenic acid, an endogenous constituent of rheumatoid arthritis synovial fluid, inhibits proliferation of synoviocytes in vitro. Rheumatol Int. 2006 Mar;26(5):422-6. Epub 2005 Oct 12. [PubMed:16220290 ]
- Amirkhani A, Heldin E, Markides KE, Bergquist J: Quantitation of tryptophan, kynurenine and kynurenic acid in human plasma by capillary liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Nov 25;780(2):381-7. [PubMed:12401365 ]
- Ilzecka J, Kocki T, Stelmasiak Z, Turski WA: Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol Scand. 2003 Jun;107(6):412-8. [PubMed:12757473 ]
- Bapurao S, Krishnaswamy K: Vitamin B6 nutritional status of pellagrins and their leucine tolerance. Am J Clin Nutr. 1978 May;31(5):819-24. [PubMed:206127 ]
- Nilsson LK, Nordin C, Jonsson EG, Engberg G, Linderholm KR, Erhardt S: Cerebrospinal fluid kynurenic acid in male and female controls - correlation with monoamine metabolites and influences of confounding factors. J Psychiatr Res. 2007 Jan-Feb;41(1-2):144-51. Epub 2006 Jan 24. [PubMed:16434056 ]
- Milart P, Sikorski R: [Kynurenic acid concentration in blood and urine during normal pregnancy]. Ginekol Pol. 1998 Dec;69(12):968-73. [PubMed:10224760 ]
- Moroni F, Russi P, Lombardi G, Beni M, Carla V: Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988 Jul;51(1):177-80. [PubMed:3379401 ]
- Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO Jr, Schwarcz R: Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988 Jun 28;454(1-2):164-9. [PubMed:3409000 ]
- Connick JH, Carla V, Moroni F, Stone TW: Increase in kynurenic acid in Huntington's disease motor cortex. J Neurochem. 1989 Mar;52(3):985-7. [PubMed:2521895 ]
- Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF: Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal Biochem. 1990 Mar;185(2):363-76. [PubMed:2339792 ]
- Baran H, Cairns N, Lubec B, Lubec G: Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 1996;58(21):1891-9. [PubMed:8637415 ]
- Beal MF, Matson WR, Storey E, Milbury P, Ryan EA, Ogawa T, Bird ED: Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J Neurol Sci. 1992 Mar;108(1):80-7. [PubMed:1385624 ]
- Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P: Age-related increase of kynurenic acid in human cerebrospinal fluid - IgG and beta2-microglobulin changes. Neurosignals. 2005;14(3):126-35. [PubMed:16088227 ]
- Muller N, Schwarz M: Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006 Oct;10(2):131-48. [PubMed:17062375 ]
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012 Jul;23(7):1258-70. doi: 10.1681/ASN.2011121175. Epub 2012 May 24. [PubMed:22626821 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
| Show more...
|---|