| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:37 UTC |
|---|
| HMDB ID | HMDB0000211 |
|---|
| Secondary Accession Numbers | - HMDB0002256
- HMDB00211
- HMDB02256
|
|---|
| Metabolite Identification |
|---|
| Common Name | myo-Inositol |
|---|
| Description | myo-Inositol is an inositol isoform. Inositol is a derivative of cyclohexane with six hydroxyl groups, making it a polyol. It also is known as a sugar alcohol, having exactly the same molecular formula as glucose or other hexoses. Inositol exists in nine possible stereoisomers, of which cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol is the most widely occurring form in nature. The other known inositols include scyllo-inositol, muco-inositol, D-chiro-inositol, L-chiro-inositol, neo-inositol, allo-inositol, epi-inositol and cis-inositol. myo-Inositol is found naturally in many foods (particularly in cereals with high bran content) and can be used as a sweetner as it has half the sweetness of sucrose (table sugar). myo-Inositol was once considered a member of the vitamin B complex and given the name: vitamin B8. However, because it is produced by the human body from glucose, it is not an essential nutrient, and therefore cannot be called a vitamin. myo-Inositol is a precursor molecule for a number of secondary messengers including various inositol phosphates. In addition, inositol/myo-inositol is an important component of the lipids known as phosphatidylinositol (PI) phosphatidylinositol phosphate (PIP). myo-Inositol is synthesized from glucose, via glucose-6-phosphate (G-6-P) in two steps. First, G-6-P is isomerised by an inositol-3-phosphate synthase enzyme to myo-inositol 1-phosphate, which is then dephosphorylated by an inositol monophosphatase enzyme to give free myo-inositol. In humans, myo-inositol is primarily synthesized in the kidneys at a rate of a few grams per day. myo-Inositol can be used in the management of preterm babies who have or are at a risk of infant respiratory distress syndrome. It is also used as a treatment for polycystic ovary syndrome (PCOS). It works by increasing insulin sensitivity, which helps to improve ovarian function and reduce hyperandrogenism. Reduced levels of myo-inositol have been found in the spinal fluid of depressed patients and levels are significantly reduced in brain samples of suicide victims. | Read more...
|---|
| Structure | O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O InChI=1S/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3-,4+,5-,6- |
|---|
| Synonyms | | Value | Source |
|---|
| (1R,2R,3S,4S,5R,6S)-Cyclohexane-1,2,3,4,5,6-hexol | ChEBI | | 1,2,3,4,5,6-HEXAHYDROXY-cyclohexane | ChEBI | | 1,2,3,5/4,6-cyclohexanehexol | ChEBI | | 1D-Myo-inositol | ChEBI | | 1l-Myo-inositol | ChEBI | | Bios I | ChEBI | | cis-1,2,3,5-trans-4,6-Cyclohexanehexol | ChEBI | | Cyclohexitol | ChEBI | | D-Myo-inositol | ChEBI | | Dambose | ChEBI | | I-inositol | ChEBI | | Inosite | ChEBI | | Inositol | ChEBI | | Ins | ChEBI | | L-Myo-inositol | ChEBI | | Meat sugar | ChEBI | | Meso-inositol | ChEBI | | Myoinositol | ChEBI | | Inosital | Kegg | | Iso-inositol | HMDB | | MI | HMDB | | Myoinosite | HMDB | | Phaseomannite | HMDB | | Rat antispectacled eye factor | HMDB | | Myo inositol | HMDB | | Vitamin b8 | HMDB | | Myo-inositol | HMDB |
| Show more...
|---|
| Chemical Formula | C6H12O6 |
|---|
| Average Molecular Weight | 180.1559 |
|---|
| Monoisotopic Molecular Weight | 180.063388116 |
|---|
| IUPAC Name | (1R,2R,3r,4S,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol |
|---|
| Traditional Name | L-inositol |
|---|
| CAS Registry Number | 87-89-8 |
|---|
| SMILES | O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3-,4+,5-,6- |
|---|
| InChI Key | CDAISMWEOUEBRE-GPIVLXJGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cyclohexanols. Cyclohexanols are compounds containing an alcohol group attached to a cyclohexane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Cyclohexanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexanol
- Sugar alcohol
- Cyclitol or derivatives
- Cyclic alcohol
- Polyol
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | Biological locationRoute of exposureSourceExogenous- Exogenous (HMDB: HMDB0000211)
Food- Food (HMDB: HMDB0000211)
Animal originHerb and spiceVegetableFruitNutCereal and cereal productPulseGourdCoffee and coffee productSoyDishTeaBaking goodBeverageAquatic originMilk and milk productUnfermented milk- Milk (Cow) (FooDB: FOOD00618)
- Cow milk, pasteurized, vitamin A + D added, 0% fat (FooDB: FOOD00889)
- Cow milk, pasteurized, vitamin A + D added, 1% fat (FooDB: FOOD00890)
- Cow milk, pasteurized, vitamin A + D added, 2% fat (FooDB: FOOD00891)
- Cow milk, pasteurized, vitamin D added, 3.25% fat (FooDB: FOOD00892)
Cocoa and cocoa product
Endogenous |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 225 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 143 mg/mL at 19 °C | Human Metabolome Project | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| myo-Inositol,1TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,1TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@H](O)[C@H](O)[C@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,1TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)[C@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,1TMS,isomer #4 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,1TMS,isomer #5 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,1TMS,isomer #6 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O | 1743.3 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #1 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H]1O | 1735.0 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #14 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #15 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #2 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H]1O | 1735.0 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #4 | C[Si](C)(C)O[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)[C@H](O)[C@H]1O | 1708.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #7 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #8 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H]1O | 1735.0 | Semi standard non polar | 33892256 | | myo-Inositol,2TMS,isomer #9 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1721.1 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #1 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #10 | C[Si](C)(C)O[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #11 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O[Si](C)(C)C)[C@H](O)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #14 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H]1O | 1780.1 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #16 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H]1O[Si](C)(C)C | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #18 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #19 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #2 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #20 | C[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H]1O | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #3 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #4 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1686.0 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #5 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #6 | C[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1780.1 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #7 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,3TMS,isomer #9 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H]1O | 1735.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #1 | C[Si](C)(C)O[C@H]1[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)[C@H](O)[C@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #11 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #12 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #13 | C[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C | 1854.5 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #14 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #15 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #2 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #3 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H]1O | 1854.5 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #5 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #6 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1804.7 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #7 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)[C@H]1O[Si](C)(C)C | 1854.5 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #8 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,4TMS,isomer #9 | C[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1858.6 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H]1O[Si](C)(C)C | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #2 | C[Si](C)(C)O[C@H]1[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #4 | C[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #5 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,5TMS,isomer #6 | C[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 1934.2 | Semi standard non polar | 33892256 | | myo-Inositol,6TMS,isomer #1 | C[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 2009.2 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@H](O)[C@H](O)[C@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)[C@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,1TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O | 2020.4 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O | 2236.9 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O | 2236.9 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)[C@H](O)[C@H]1O | 2193.7 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O | 2236.9 | Semi standard non polar | 33892256 | | myo-Inositol,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2206.2 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2511.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H]1O | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2456.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2511.9 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H]1O | 2490.3 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2735.3 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O | 2735.3 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2713.4 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2735.3 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,4TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2733.1 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H]1O[Si](C)(C)C(C)(C)C | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]1[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,5TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2931.4 | Semi standard non polar | 33892256 | | myo-Inositol,6TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3172.1 | Semi standard non polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (6 TMS) | splash10-014j-0953000000-1571a0577293e96091b1 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (6 TMS) | splash10-00kb-0932000000-96ddc21293431d746ad2 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00kb-0942000000-b78076224adf13bade25 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00kb-0942000000-42c4e40d04b4f306535d | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (6 TMS) | splash10-00di-8942000000-f5d7abd38d6ff547a0a9 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-MS (6 TMS) | splash10-066r-0975000000-df7304c12b6c9bdb0f76 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Non-derivatized) | splash10-014j-0953000000-1571a0577293e96091b1 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Non-derivatized) | splash10-00kb-0932000000-96ddc21293431d746ad2 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Non-derivatized) | splash10-00kb-0942000000-b78076224adf13bade25 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-EI-TOF (Non-derivatized) | splash10-00di-8942000000-f5d7abd38d6ff547a0a9 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - myo-Inositol GC-MS (Non-derivatized) | splash10-066r-0975000000-df7304c12b6c9bdb0f76 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (Non-derivatized) - 70eV, Positive | splash10-03dr-9700000000-18a13ab4150e6ebb20cd | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (6 TMS) - 70eV, Positive | splash10-00fr-9001040000-00e67b437c4329aee95d | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - myo-Inositol GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Quattro_QQQ 10V, N/A-QTOF (Annotated) | splash10-0a59-3900000000-96084971c3e13a1e7451 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Quattro_QQQ 25V, N/A-QTOF (Annotated) | splash10-001i-9000000000-1de17d7f53ca727e6d72 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Quattro_QQQ 40V, N/A-QTOF (Annotated) | splash10-0zh9-9000000000-bef910214341056a78aa | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 2V, negative-QTOF | splash10-004i-0900000000-41a49af846b1636763b5 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 2V, negative-QTOF | splash10-004i-0900000000-06f9ecfccf30d40ef204 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 3V, negative-QTOF | splash10-004i-0900000000-bca88f6782a8ed386223 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 4V, negative-QTOF | splash10-004i-1900000000-500ab195d69568b8678b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 5V, negative-QTOF | splash10-01ti-5900000000-a1657b54c985e2be1d19 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 6V, negative-QTOF | splash10-000i-9600000000-325cbb8dbfdeafb2b31f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 8V, negative-QTOF | splash10-000i-9200000000-9145a4766351204e723a | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 9V, negative-QTOF | splash10-000i-9100000000-2bbbcfa4733b749ec768 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 10V, negative-QTOF | splash10-000i-9000000000-de0ec8731a152b612bb9 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 12V, negative-QTOF | splash10-0079-9000000000-41d049d1feef94e190d4 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 14V, negative-QTOF | splash10-059i-9000000000-796e728daf28d5122dee | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 15V, negative-QTOF | splash10-059i-9000000000-8643e206319f3008a8d4 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 18V, negative-QTOF | splash10-0abi-9000000000-98e61f19bc2842f2e97b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol Orbitrap 20V, negative-QTOF | splash10-0ab9-9000000000-29aa062fc4d5341167b6 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol n/a 12V, negative-QTOF | splash10-03di-1900000000-5a2897fd032a959918b3 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - myo-Inositol n/a 12V, negative-QTOF | splash10-000i-9000000000-71c4786b7034d5a0a0a9 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 10V, Positive-QTOF | splash10-001i-0900000000-5cb39f92646251665ebf | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 20V, Positive-QTOF | splash10-001i-0900000000-2aeb7708f31c772e2d95 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 40V, Positive-QTOF | splash10-08gi-8900000000-74f89711a1c6891808a4 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 10V, Negative-QTOF | splash10-004i-0900000000-8b68ff47f846a8ca1ddd | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 20V, Negative-QTOF | splash10-004i-1900000000-03ef8572289795275d9c | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - myo-Inositol 40V, Negative-QTOF | splash10-0570-9300000000-950a37bd331028801e9f | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Breast Milk
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Sweat
- Urine
|
|---|
| Tissue Locations | - Basal Ganglia
- Brain
- Fibroblasts
- Intestine
- Kidney
- Neuron
- Placenta
- Prostate
- Testis
|
|---|
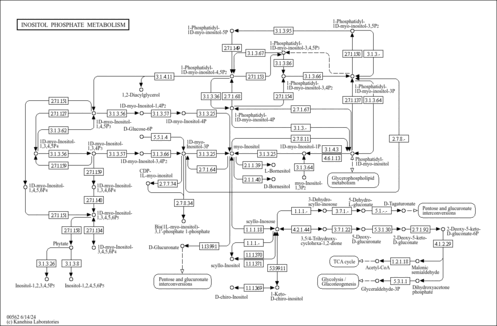
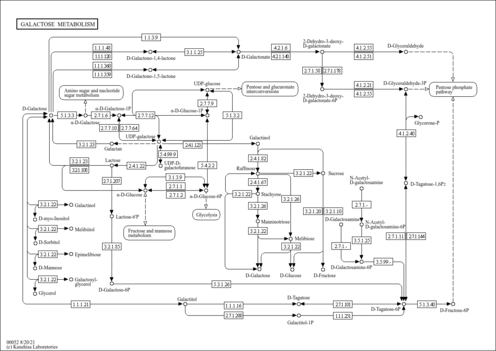
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 17.1 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | <55.556 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 24.0 +/- 7.8 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 23.0 +/- 8.0 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 30.0 (21.0-49.0) uM | Adult (>18 years old) | Both | Normal | | details | | Breast Milk | Detected and Quantified | 865 +/- 177 uM | Adult (>18 years old) | Female | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 174.0 +/- 31.0 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 84 +/- 40 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 158.8 +/- 46.07 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 138.3 +/- 24.4 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 133.0 (111.0-155.0) uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Not Available | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Not Specified | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 21.78 +/- 14.28 uM | Adult (>18 years old) | Both | Normal | | details | | Sweat | Detected and Quantified | < 10 uM | Adult (60 years old) | Male | Normal | | details | | Sweat | Detected and Quantified | < 10 uM | Adult (40 years old) | Male | Normal | | details | | Sweat | Detected but not Quantified | Not Quantified | Adult | Both | Normal | | details | | Urine | Detected and Quantified | 12.6 (5.1-15.3) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 18.8 +/- 2.9 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 490 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 22.4 (7.9-36.1) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 52.8 +/- 47.7 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 40.555 +/- 25.479 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 6.11 +/- 6.25 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Schizophrenia | | details | | Blood | Detected and Quantified | 522.222 +/- 383.333 uM | Adult (>18 years old) | Both | Uremia | | details | | Blood | Detected and Quantified | 20.0 (23.0–24.0) uM | Adult (>18 years old) | Both | Ribose-5-phosphate isomerase deficiency | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 158.8 +/- 27.2 uM | Adult (>18 years old) | Both | Alzheimer's disease | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 158.8 +/- 27.2 uM | Not Specified | Not Specified | Alzheimer's disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected and Quantified | 20.33 +/- 13.70 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Cachexia | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected and Quantified | 42.109 +/- 37.05 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Ribose-5-phosphate isomerase deficiency |
|---|
- Huck JH, Verhoeven NM, Struys EA, Salomons GS, Jakobs C, van der Knaap MS: Ribose-5-phosphate isomerase deficiency: new inborn error in the pentose phosphate pathway associated with a slowly progressive leukoencephalopathy. Am J Hum Genet. 2004 Apr;74(4):745-51. Epub 2004 Feb 25. [PubMed:14988808 ]
| | Schizophrenia |
|---|
- Xuan J, Pan G, Qiu Y, Yang L, Su M, Liu Y, Chen J, Feng G, Fang Y, Jia W, Xing Q, He L: Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res. 2011 Dec 2;10(12):5433-43. doi: 10.1021/pr2006796. Epub 2011 Nov 8. [PubMed:22007635 ]
| | Uremia |
|---|
- Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W: Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003 May;63(5):1934-43. doi: 10.1046/j.1523-1755.2003.00924.x. [PubMed:12675874 ]
| | Alzheimer's disease |
|---|
- Shetty HU, Holloway HW, Schapiro MB: Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996 Feb;42(2):298-302. [PubMed:8595727 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Cachexia |
|---|
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I: HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009 Jan;37(Database issue):D603-10. doi: 10.1093/nar/gkn810. Epub 2008 Oct 25. [PubMed:18953024 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | - 608611 (Ribose-5-phosphate isomerase deficiency)
- 181500 (Schizophrenia)
- 104300 (Alzheimer's disease)
- 114500 (Colorectal cancer)
- 610247 (Eosinophilic esophagitis)
|
|---|
| External Links |
|---|
| DrugBank ID | DB13178 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB010547 |
|---|
| KNApSAcK ID | C00001164 |
|---|
| Chemspider ID | 10239179 |
|---|
| KEGG Compound ID | C00137 |
|---|
| BioCyc ID | MYO-INOSITOL |
|---|
| BiGG ID | 33990 |
|---|
| Wikipedia Link | Inositol |
|---|
| METLIN ID | 5221 |
|---|
| PubChem Compound | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17268 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | INOST |
|---|
| MarkerDB ID | MDB00000101 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Iselin, Beat M. Synthesis of inositol-5-monophosphoric acid and scyllitol monophosphoric acid. Journal of the American Chemical Society (1949), 71 3822-5. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Shetty HU, Holloway HW, Rapoport SI: Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem. 1995 Jan 1;224(1):279-85. [PubMed:7710082 ]
- Kusmierz J, DeGeorge JJ, Sweeney D, May C, Rapoport SI: Quantitative analysis of polyols in human plasma and cerebrospinal fluid. J Chromatogr. 1989 Dec 29;497:39-48. [PubMed:2625478 ]
- Gryz EA, Galicka-Latala D, Szczudlik A, Sieradzki J: [Etiopathogenesis of diabetic neuropathy]. Przegl Lek. 2000;57(12):727-31. [PubMed:11398597 ]
- Kim MO, Im JH, Choi CG, Lee MC: Proton MR spectroscopic findings in paroxysmal kinesigenic dyskinesia. Mov Disord. 1998 May;13(3):570-5. [PubMed:9613757 ]
- Wuarin-Bierman L, Zahnd GR: Current aspects of research on the pathogenesis of diabetic neuropathy. Diabete Metab. 1986 Dec;12(6):319-24. [PubMed:3028879 ]
- Reznek RH, Salway JG, Thomas PK: Plasma-myoinositol concentrations in uraemic neuropathy. Lancet. 1977 Mar 26;1(8013):675-6. [PubMed:66475 ]
- Scully SE, Stebner FC, Yoest SM: Magnetic resonance spectroscopic findings in neuro-Behcet disease. Neurologist. 2004 Nov;10(6):323-6. [PubMed:15518598 ]
- Cordoba J, Hinojosa C, Sampedro F, Alonso J, Rovira A, Quiroga S, Esteban R, Guardia J: Usefulness of magnetic resonance spectroscopy for diagnosis of hepatic encephalopathy in a patient with relapsing confusional syndrome. Dig Dis Sci. 2001 Nov;46(11):2451-5. [PubMed:11713951 ]
- Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN: Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999 Sep 11;53(4):782-9. [PubMed:10489041 ]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, Kido D: Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res. 2004 Oct;56(4):630-8. Epub 2004 Aug 4. [PubMed:15295080 ]
- Servo C, Pitkanen E: Variation in polyol levels in cerebrospinal fluid and serum in diabetic patients. Diabetologia. 1975 Dec;11(6):575-80. [PubMed:1205026 ]
- Utriainen M, Komu M, Vuorinen V, Lehikoinen P, Sonninen P, Kurki T, Utriainen T, Roivainen A, Kalimo H, Minn H: Evaluation of brain tumor metabolism with [11C]choline PET and 1H-MRS. J Neurooncol. 2003 May;62(3):329-38. [PubMed:12777086 ]
- Grzelec H: [Pathogenesis and treatment of diabetic neuropathy]. Neurol Neurochir Pol. 1991 Jul-Aug;25(4):477-84. [PubMed:1666426 ]
- Ernst T, Itti E, Itti L, Chang L: Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. J Magn Reson Imaging. 2000 Dec;12(6):859-65. [PubMed:11105023 ]
- Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L: Role of myoinositol in regulation of surfactant phospholipids in the newborn. Early Hum Dev. 1985 Jan;10(3-4):245-54. [PubMed:3838720 ]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA: A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004 Dec;23(4):1336-47. [PubMed:15589098 ]
- Rysz J, Bartnicki P, Blaszczak R, Kujawski K, Cialkowska-Rysz A, Olszewski R, Markuszewski L: [Anti-inflammatory action of myoinositol in renal insufficiency]. Pol Merkur Lekarski. 2006 Feb;20(116):180-3. [PubMed:16708635 ]
- Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, Richards TL, Kwok EW, Kolson DL, Simpson D, Tang CY, Schifitto G, Ketonen LM, Meyerhoff DJ, Lenkinski RE, Gonzalez RG, Navia BA: A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003 Jun;17(6):625-33. [PubMed:12766890 ]
- Galasko GT, Bao Y, Broomfield SJ, Hooper NM, Turner AJ, Larner J: Circulating factors and insulin resistance. I. A novel myoinositol 1,2-cyclic phosphate phosphoglycan insulin antagonist from human plasma is elevated in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995 Aug;80(8):2419-29. [PubMed:7629237 ]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA: Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004 Nov;23(3):928-35. [PubMed:15528093 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
| Show more...
|---|